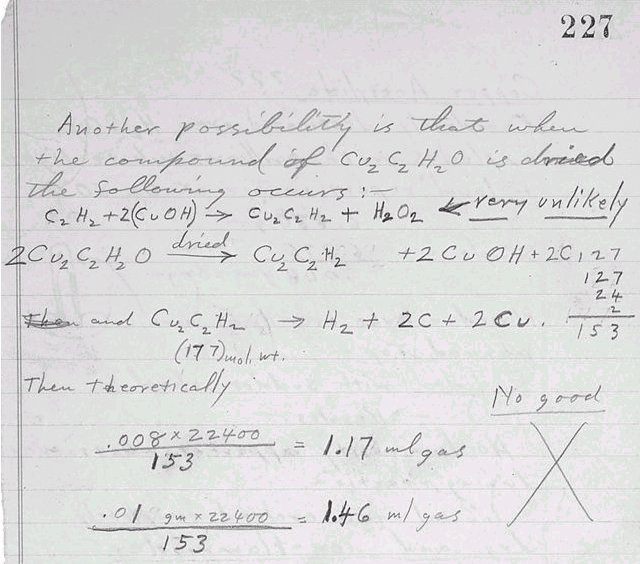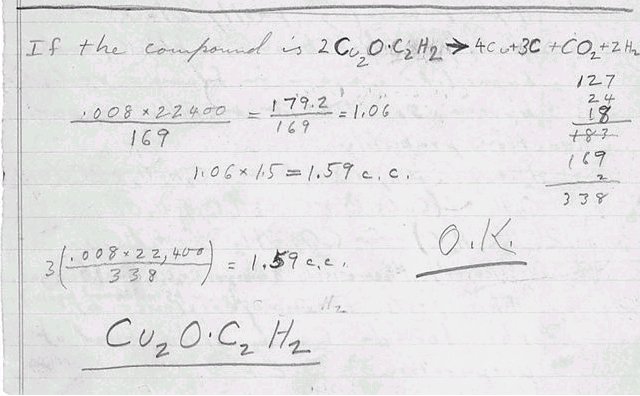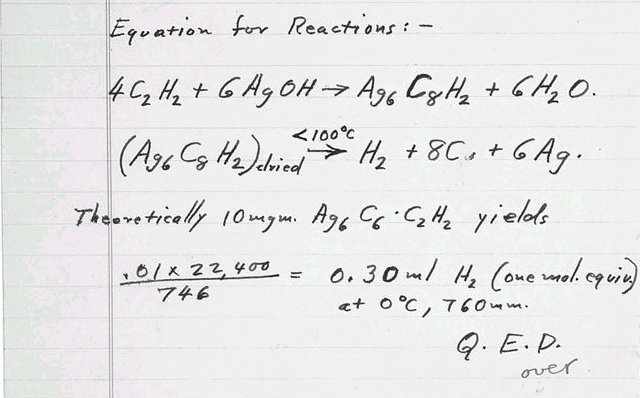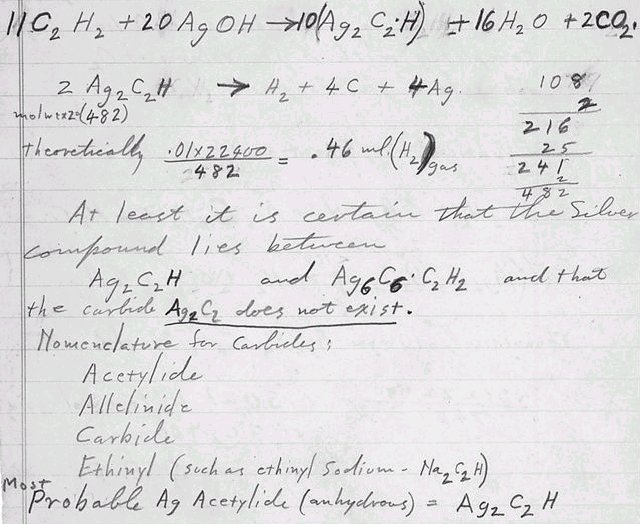|
또다른 가능성은 Cu2C2H2O의
복합물이 건조할 때, 다음이 발생한다는
것이다:
C2H2+2(Cu2OH)
------> Cu2C2H2+H2O2
<=== 아주 가망없는
건조
2Cu2C2H2O
----------> Cu2C2H2
+ 2CuOH + 2C 127
127
24
2
그리고
Cu2C2H2
----------> H2
+ 2C + 2Cu 153
(177)
mol.중량
그러면, 이론적으로,
좋지
않다.
0.008 x 22400 = 1.17 ㎖ 기체
153
0.01 gram x 22400 = 1.46 ㎖ 기체
153
만약에 복합물이 2Cu2O•C2H2
---> 4Cu + 3C + CO2 +
2H2O 이면,
0.008 x 22400 = 179.2 =
1.06 127
169 169 24
18
183
1.06 x 1.5 = 1.59 cc 169
2
338
3(0.008 x 22,400) = 1.59
cc 옳다.
338
0.01
gram x 22400 = 1.46 ㎖ 기체
153
Cu2O•C2H2
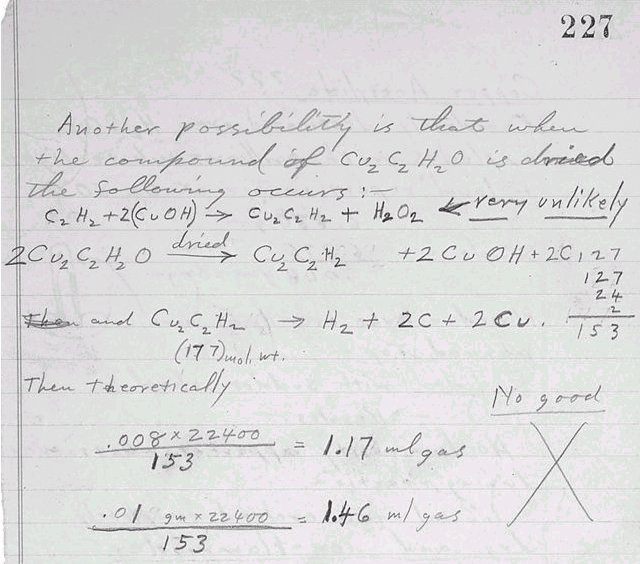
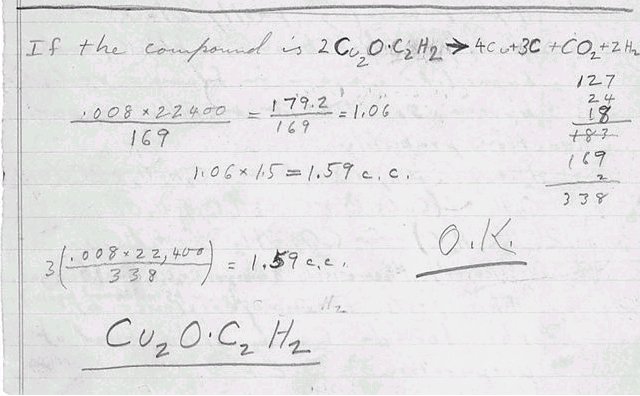
Copper Acetylide
???? (Copper와
Acetylene의 화합물질로 강한 폭발성을
지님)
황산(H2SO4)로 완전히 건조된
60℃의
공기 중에서 밝은 백색광과 큰
폭발음을
내면서 폭발한다.
6.878
그램
예: -6.870
-자체중량
0.008
그램 = 8 밀리그램
보이는
바와 같이, 수은(Hg) 위에 관을
놓는다.
상부가 데워진다.
갑자스러운
쿵 소리와 함께 폭발한다.
-
결과 -
반응을 일으키는
동안 뚜렷한 어떠한 발광도 없다.
1.6
㎖의 완전히 건조된 불연성 기체가
방출되었다.
남은 침전물 -
부분적으로 검정색 - 부분적으로
적색을 띤 갈색 - 검정색 분말은
화염 속의 탄소와 같이 불탄다
- 금속 구리(copper)과 유사한
금속성 광택(luster)을
지닌 적색을 띤 갈색.
그러므로, 반응들은
아마도,
C2H2+
2(CuOH)
--------> Cu2C2H2O
+ H2O
2(Cu2C2H2O)
----------> CO2+
2H2
+ 3C + 4Cu
(건조된)
더 잘 쓰면, Cu2O•C2H2
그러므로,
이론적으로 8 그램의 Cu2C2H2O
----------> 1.6 cc의
기체.
이것은, 증명하였듯이,
이 준비된 방법에 의해서 식 Cu2C2은
존재하지 않는다는 것을 증명한다.
끝


탄화은(Silver Acetylide)
- 황산(H2SO4)로 철저히 건조된
(문지르거나 수은(Hg)에
근접하면 공기 중에서 격렬하게
폭발한다)
수은 위에 있는
버너(burner)로 가열한 밀봉된
관에 들어있는 10 밀리그램은
백색의 불꽃을 지닌 밝은 발광을
내면서 폭발한다. 폭발을 방지하기
위하여 수은을 천천히 그리고
부드럽게 부어야 한다. 잔류기체는
0.3 cc의 용량을 지니며 완전히
건조된다. 많은 양의 비정질(amorphous)
탄소가 관 전체로 흩어졌다. 기체는
50% 수산화캘시엄(KOH) - 이산화탄소(CO2)가
존재하지 않는다) - 에 흡수되지
않음을 보여주었다.
공기 중에서
약간의 빛을 지닌 화염을
내면서 인화한 기체는 O2
용적의 1/2을 지닌 완벽한 H2O
액체를 형성한다. 그러므로, 기체는
순수한 H2이다.
반응식들:
4C2H2+
6AgOH
------> Ag6C8H2
+ 6H2O
<
100℃
(Ag6C8H2)건조된
-------> H2 +
8C + 6Ag
이론적으로 10 밀리그램 Ag6C6•C2H2는,
증명하였듯이,
0.01 x
22,400 = 0 ℃, 760 mm에서
0.30 ㎖ H2(1 mol.과
같다)
746
끝

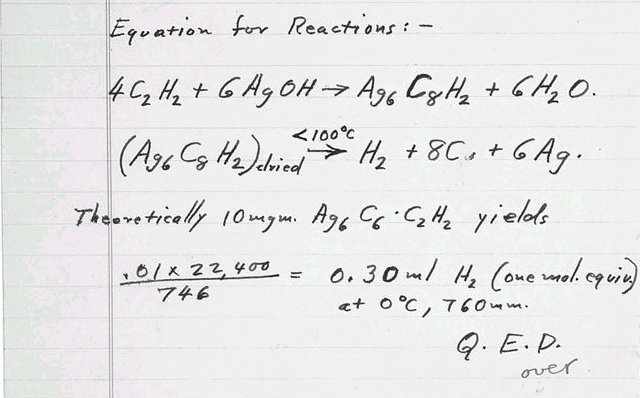
본래의 복합물의 일부는
관의 상부에서 관찰된 바와 같이 폭발하지
않았던 것으로 알려져 있다. 이것은
아마 격렬한 폭발과 밀폐된 포장의
??? 장해나 부반응으로 인한 불순물
때문일 것이다.
그러므로, 탄화은의
복합물은 Ag2C2•H일
가능성이 아주 높으며 그 반응은
다음과 같다:
11C2H2
+ 20AgOH -------> 10(Ag2C2•H)
+ 16H2O + 2CO2
2Ag2C2H
-------> H2
+ 4C + 4Ag 108
mol 중량 x 2 (482)
2
216
이론적으로
0.01 x 22400 = 0.46 ㎖
(H2)기체 25
482
241
2
482
은 복합물은 적어도
2Ag2C2H와
Ag6C6•C2H2사이에
놓이며 탄화물 Ag2C2는
존재하지 않는다는 것은 확실하다.
탄화물들에
대한 학명은;
Acetylide
Allelinide
Carbide
Ethinyl
(ethinyl sodium - Na2C2H
과 같은)
거의 틀림없이
Ag Acetylide (무수) = Ag2C2H

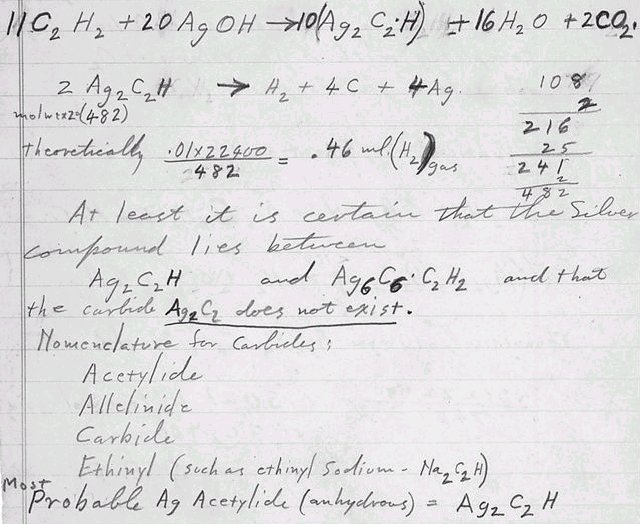
|